Research Projects

Mechanistic Investigations of Dehaloperoxidase – The First Multifunctional Catalytic Hemoglobin
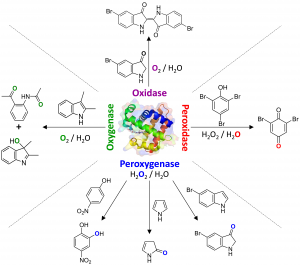
The enzyme known as dehaloperoxidase-hemoglobin has provided us with a recent example of a multifunctional enzyme that has the potential to significantly advance our understanding of the protein structure-function correlation. Of course, structure is related to function, but the question is whether, or how, structure uniquely defines function. In DHP, it appears that the substrate itself plays a pivotal role in determining which activity (peroxidase, peroxygenase, oxidase and/or oxygenase) the enzyme performs. Altogether, this intriguing functional switch in the presence of different substrates may be triggered by the physical or electronic properties of the substrate, its binding orientation in the active site, or by a change in the protein’s redox properties. In essence, the various substrates alter the enzymatic function of the catalytic globin in much the same way allosteric effectors would structurally shift the equilibrium of a cooperative hemoglobin. Thus, DHP provides a unique and advantageous platform for deeply probing mechanistic questions related to the protein environment: by simply changing the substrate, the unique set of protein-substrate interactions specific to each of the five different heme activities of DHP can be studied, enabling us to pose questions related to multiple activities across the heme protein superfamily using just a single enzyme. Moreover, the relationship of DHP to other hemoglobins, peroxidases and peroxygenases aids in establishing new paradigms of protein structure-function relationships relevant to multifunctional proteins, complementing those established for monofunctional enzymes. More broadly, the knowledge gained through the studies performed on DHP over the past two decades advances our understanding of catalytic globins, helps to elaborate the structural features that lead to activity differentiation across heme proteins (and across metalloenzymes more generally), and furthers the comparison of the structure-function correlation in enzymes of marine origin in relation to terrestrial or bacterial ones.
Antimicrobial Materials for Combatting Hospital-Acquired Infections (HAIs) and COVID-19
The Ghiladi group is developing two comprehensive antimicrobial strategies wherein polymeric materials are capable of rapidly inactivating a wide range of dangerous pathogens and thus mitigating the global spread of infectious diseases, including COVID-19. One approach utilizes photodynamic molecules or nanoparticles that, upon chemical attachment onto polymer surfaces or physical incorporation into bulk polymers or polymer coatings, generate toxic reactive oxygen (primarily singlet oxygen) in the presence of incoherent visible light and inactivate Gram-positive/negative bacteria responsible for hospital-acquired (nosocomial) infections, as well as viruses (such as influenza and human coronavirus) and toxic fungi, on the polymer surface. After an exposure time of 30-60 min at modest light intensities, the lowest inactivation levels recorded range from 99.89% for bacteria to 99.95% for viruses and 99.58% for fungi, whereas the maximum levels achieved lie below the minimum detection level corresponding to 99.9999+% inactivation. These additives remain active for over a month, which makes them ideal for personal protective equipment (PPE), including face masks/shields, medical clothing and hospital linen, to not only improve personal safety but also reduce the level of solid waste associated with the healthcare industry.
A second, independent strategy is based on a family of anionic nanostructured polymers, such as self-assembled block polymers that can be recycled and reused. Fundamentally different from conventional cationic polymer films or gels and unlike dissolved anionic polymers frequently added as antivirals to aqueous suspensions intended for drug delivery, these antimicrobial surfaces function on the principle of a significant pH drop (to below 1) at the polymer surface. In this case, hydrophilic nanoscale channels transport subsurface protons from sulfonic acid groups located on the polymer backbone, while nonpolar moieties ensure that the polymer films/coatings remain mechanically stable. While several different materials have been shown to exhibit this behavior, two of particular interest are BIAXAMTM, manufactured for water purification and moisture management, and NAFIONTM, employed in fuel cells and electroactive polymers. In addition to inactivating the above pathogens to the minimum detection level in just 5 minutes, they also kill highly contagious SARS-CoV-2 (99.9% in 5 min), antibiotic-resistant MRSA (99.9999+% in 5 min) and frequently fatal C. difficile (99.99% in 1 min in the transmissible vegetative state and ~90% in 60 min in the self-protecting spore state).
These two mechanistically different antimicrobial polymer strategies have been published (or submitted for publication) in the open literature, addressed in patents, featured in press releases, and transferred to commercial manufacturing. Together, they provide continuous, expedient and economical inactivation to combat the growing global threat of highly contagious and drug-resistant pathogens, prevent the transmission of a wide range of current, as well as future, infectious microbes, reduce the financial burden on the healthcare industry, and ultimately save lives worldwide.
